The Landscape of Lens Design: From Theory to Practice
An interview with IOT's Director of Clinical Research

The Landscape of Lens Design: From Theory to Practice
An interview with IOT's Director of Clinical Research
In the optical industry, it can be easy to get caught up in the business side of our world and forget the essence of what we do: create the most innovative lens designs that best help our patients solve their vision needs. At IOT, we know this and have an extensive validation process through clinical trials with glasses wearers, allowing us to create unique solutions for many different types of eyes and lifestyles.
The importance of significant R&D and wearer trials cannot be understated. As optical nerds, we often get caught up in the “math” behind our designs, as it is exciting scientific innovation – however, we must not forget that the success of a lens design is subjective. Human discernment is a huge factor in the success or failure of a new lens. Connecting the expectations of patients with our groundbreaking technologies is an essential part of what we do.
While IOT may not be the largest lens design company out there, our R&D team and intensive wearer trials aim to create the perfect blend of user experience and scientific research.
An interview with Dr. Eva Chamorro

I recently had the amazing opportunity to interview Dr. Eva Chamorro, the Director of Clinical Research for IOT in Madrid. Eva earned her degree in optometry (Masters, Research and Optometry) as well as a Master’s degree in Low Vision. Additionally, she earned her Doctorate/Ph. D with vast research in light, and parts of the electromagnetic spectrum.
Eva started at IOT in 2013 in the Technical Department where she stayed for 5 years, until starting the direction of the Clinical Research Team. She notes: ‘Working within the Technical Team was a really great opportunity for me to understand better how the ophthalmic/optics industry works, and to hear from lens manufacturers to better understand their wants, needs, and pains.
In the Clinical Research Department, they work mainly in the design of new ophthalmic lenses, but also in the development of tools that help eye care professionals be more successful in dispensing IOT designs. The main responsibility of the team is to test every product by means of controlled wearer trials. This allows our scientists to gather user feedback and provides vital information about how to develop new and better products.
Approach to wearer trials
Dr. Chamorro shared that, even though IOT is a relatively young company, it has been passionate about wearer trials from the start. This has led to significant growth, including building a specialized clinical trials team while also investing in spaces to conduct research, using specialized testing tools like aberrometers and eye-tracking devices to study the patients’ vision in-depth.
She states: “We have a team of highly qualified optometrists, mathematicians, and administrators who are dedicated exclusively to do wearer trials.”
According to Dr. Chamorro, the knowledge and experience gained through these trials over the years yields a much better understanding of how the lenses and eyes work together. And this is not a small statistical sample: IOT conducts approximately 20 wearer trials per year, attending to around 600 patients.
Including during the pandemic! Eva states: “Even during 2020, the year mostly affected in Spain with the Covid pandemic, we were conducting wearer trials. Of course, we had to adapt our methodology. But, during that year we attended to 1300 patients.”
Read our recently published MyoLess clinical trial
Members of our Clinical Trials Department have just recently published a new clinical trial, titled "Effectiveness of a Spectacle Lens with a Specific Asymmetric Myopic Peripheral Defocus: 12-Month Results in a Spanish Population". The study validated the effectiveness of MyoLess, demonstrating a notable 39% reduction in axial length growth in children.
A user-centered lens design process
For our clinical trial team, patient feedback that comes up during clinical trials is the most important point to ensure that our products meet their visual needs. Wearer trials allow them to put the user at the center of the design process, thus creating and adapting the designs based on the wearers’ feedback.
Likewise, the Clinical Research Department at IOT is active beyond the borders of their headquarters in Madrid. Every year, they participate in several congresses and conferences that relate to vision science. Even on the day of Dr. Chamorro’s interview, two team members were presenting posters explaining methods of evaluating PAL performance by using eye-tracking devices. In addition to publishing periodical papers in scientific journals, they also participate as visiting instructors for opticianry and optometry colleges and universities.
In science, subjective wearer trials don´t always match the objective hypothesis. This is not necessarily a bad thing: "Sometimes, we get positive results, and the trial confirms our hypothesis. But we also see unexpected results. It is common to think that unexpected results are bad. But for us, it is not. Unexpected results help us to advance and to improve, because we need to understand the reasons behind those results, and to find better solutions that help us achieve our goal. Most of the time, this leads us to creating new hypotheses that help us design and develop better technologies."
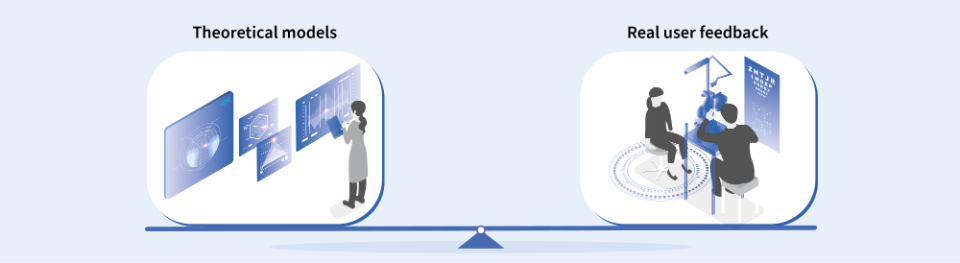
Balancing clinical trials and theoretical models
When asked about the overall learning experience throughout the years, Eva shared:
“When we study at university, one of the most common things we hear is ’the eye is a complex organ’. And it really is. The perception of the eye has a subjective component, and we cannot presuppose anything. In all wearer trials we found patients that don’t perceive differences between lenses, or patients with unexpected answers. This could be due to the adaptation capacity of the individual, previous experience with other lenses or even emotional factors. In this sense, close monitoring of the patient helps us to better interpret the data. Moreover, working as a team becomes essential here. By sharing and discussing our findings, we can draw accurate and meaningful conclusions together.”
When asked to compare the importance of real-world user feedback through wearer trials to that of theoretical models in designing lenses that satisfy patients' needs, Dr. Chamorro emphasized that both are equally vital, assigning them an equal weight of 50/50.
“We need both. Theoretical models alone are incomplete, because lens performance has a subjective component, and this can only be tested by controlled wearer trials that collect the feedback of the users. However, if we only based our development and analysis in wearer trials, the process will not be effective because we would have to work without a good hypothesis. That could lead to inconclusive and/or difficult-to-understand results.”
Thank you so much to Dr. Eva Chamorro, for adding an abundance of knowledge and understanding to our professions, for the betterment of our patients!
Let's transform vision care together. Complete the form below and we'll put you in touch with a sales representative.